The Anonym tab is a dedicated version of the batch format conversion which enforces de-identification of the input images. See the description above for an explanation of the working procedure.
ANONYMIZE brings up the configuration dialog window below.
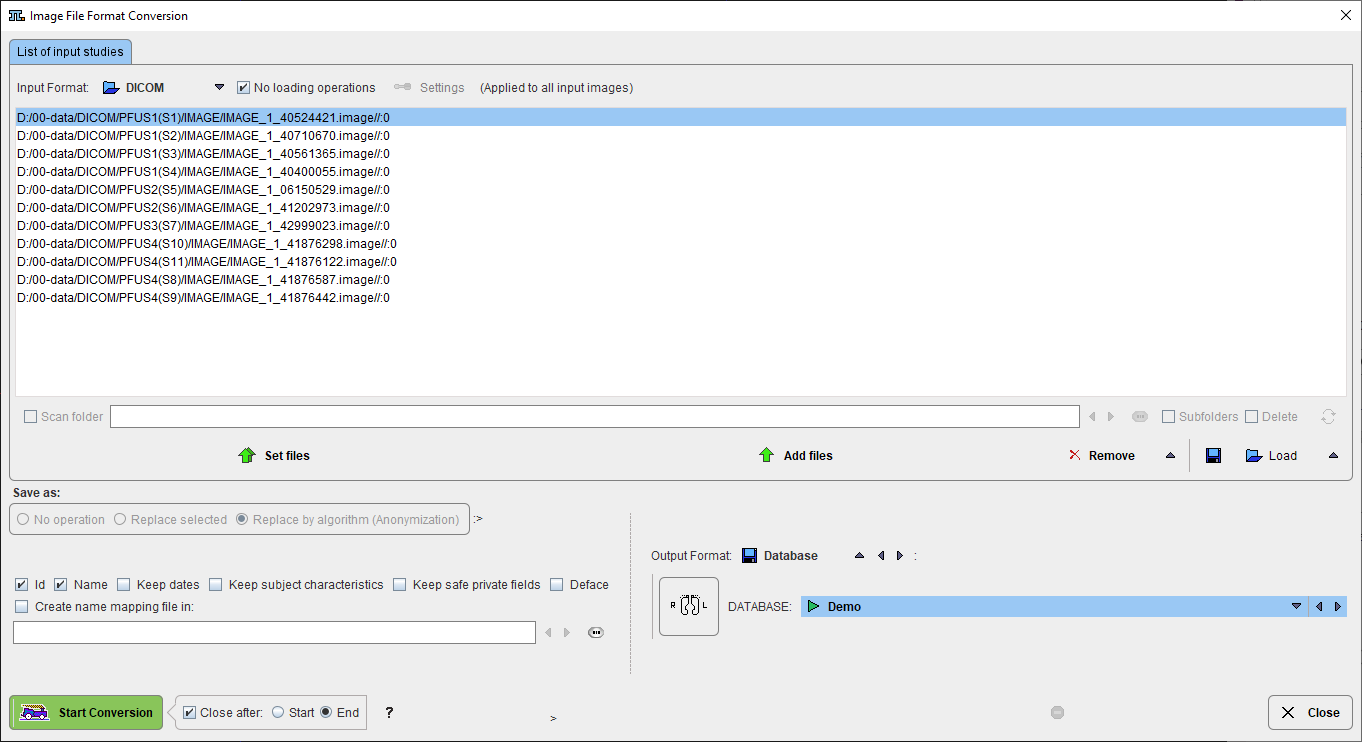
De-identification of the images is performed according to DICOM PS3.15 Annex E. It implements the E.2 "Basic Application Level Confidentiality Profile" intended for use in Clinical Trials. It is extremely conservative and removes all information related to identity and demographics of the subject, his family, personnel and the organization. The following options allow keeping a part of this information.
Id / Name |
This option is useful if subject name and id were already replaced and should be kept. |
Keep dates |
Check enabled corresponds to "Retain Longitudinal Temporal Information with Full Dates" in section E.3.6 in the Annex. In this case the dates are not modified. Check disabled corresponds to "Retain Longitudinal Temporal Information with Modified Dates". In this case the dates and times will be changed in such a way that calculations such as SUV still work and the temporal relation between longitudinal acquisitions should still be maintained. |
Keep subject characteristics |
Check enabled corresponds to "Retain subject Characteristics" in section E.3.7 in the Annex and concerns information about sex, size, age, status. Without this option, SUV calculation will not be possible. |
Keep save private fields |
Private elements considered as safe in DICOM PS3.15 Annex E or indicated as safe in the object will be kept. |
Deface |
Removes facial features from the image. |
Create name mapping file |
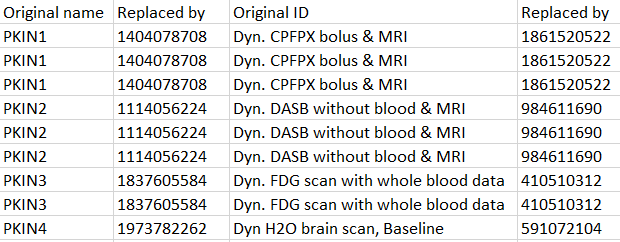
With this option the export procedure generates a text file deident.map which lists the original names/IDs with their replacement values. Note that a hashing procedure generates the same values if the names and IDs are consistent among studies.
|