In the last years the analysis of the amyloid deposition in the brain using composite cortical VOI became common. There have been slight variations regarding which brain regions are included in amyloid cortical composites. The most common amyloid cortical composite includes frontal, temporal, and parietal cortices, including cingulate regions as well as precuneus [1-8]. These amyloid cortical composite regions have been published and validated using various amyloid PET radioligands such as and not limited to [11C]PIB, [18F]Florbetapir (Amyvid, Eli Lilly), [18F]flutemetamol (Vizamyl, GE Healthcare) and [18F]florbetaben (NeuraCeq, Piramal Pharma).
Various reference regions have been used to calculate standardized uptake value ratio (SUVr) such as whole cerebellum, cerebellar grey matter, pons, brainstem and white matter. In addition, an amyloid cortical composite combined with a composite reference region (e.g., whole cerebellum, brainstem/pons and white matter) has been shown to improve the longitudinal measures of amyloid [3, 9].
Thus, we provide our users the most common pre-defined amyloid cortical composite template including various reference regions used for calculating SUVr.
VOI Atlas
The VOI atlas Amyloid Cortical Composite can be selected in the list of included VOI atlases. The corresponding files can be found in the resources/templates/voitemplates/Amyloid Cortical Composite directory.
The capture below illustrates the VOIs structures overlaid on the gray matter probability map in MNI space.
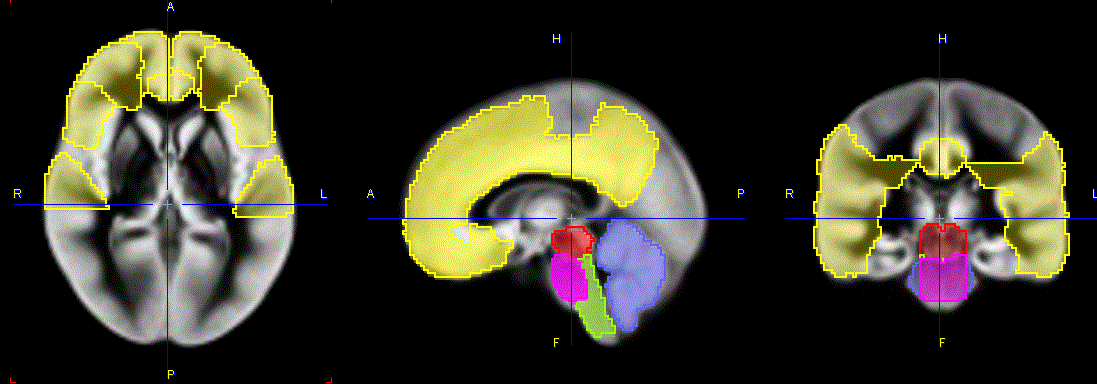
There are 5 brain structures included in the Amyloid Cortical Composite atlas. In comparison to the the original Hammers N30R83 atlas the Cortical Composite and the Cerebellum listed structures were created pooling the specified regions. The even label numbers denote left structures, the uneven numbers right structures in the Hammers N30R83 atlas. The Midbrain, Medulla Oblongata and the Pons were manually segmented from the original Hammers N30R83 Brainstem structure. The segmentation was performed in such manner to avoid contour overlapping in the new structures.
28; 29 11; 12 62; 63 24; 25 |
Middle frontal gyrus Superior temporal gyrus posterior part Superior parietal gyrus Cingulate gyrus (gyrus cinguli), anterior part |
17; 18 |
Cerebellum |
19 |
Structure of the Brainstem |
19 |
Structure of the Brainstem |
19 |
Structure of the Brainstem |
References
1.Hutton, C., et al., Quantification of [18F]florbetapir PET: comparison of two analysis methods. Eur J Nucl Med Mol Imaging, 2015. 42(5): p. 725-32.
2.Johnson, K.A., et al., Florbetapir [18F]AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement, 2013. 9(5 Suppl): p. S72-83.
3.Landau, S.M., et al., Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med, 2013. 54(1): p. 70-7.
4.Liu, E., et al., Amyloid-beta [11C]PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology, 2015. 85(8): p. 692-700.
5.Nayate, A.P., et al., Use of Standardized Uptake Value Ratios Decreases Interreader Variability of [18F]Florbetapir PET Brain Scan Interpretation. AJNR Am J Neuroradiol, 2015. 36(7): p. 1237-44.
6.Ostrowitzki, S., et al., Mechanism of amyloid removal in subjects with Alzheimer disease treated with gantenerumab. Arch Neurol, 2012. 69(2): p. 198-207.
7.Sevigny, J., et al., Amyloid PET Screening for Enrichment of Early-Stage Alzheimer Disease Clinical Trials: Experience in a Phase 1b Clinical Trial. Alzheimer Dis Assoc Disord, 2016. 30(1): p. 1-7.
8.Wolk, D.A., et al., Amyloid imaging in Alzheimer's disease: comparison of florbetapir and Pittsburgh compound-B positron emission tomography. J Neurol Neurosurg Psychiatry, 2012. 83(9): p. 923-6.
9.Landau, S.M.a.J., W., Florbetapir processing methods. ADNI PET core, 2015.